In Kjeldahl's Method Potassium Sulphate Acts As . potassium sulphate is usually added to increase the boiling point of the medium. three basic steps are required to perform the kjeldahl analysis: The acid digestion mixture is diluted and made strongly alkaline with naoh, liberating nh3 as follows:. The method entails converting all nitrogen in a weighed sample to ammonium sulphate via sulfuric acid digestion, alkalizing the solution, and determining. This method includes a wet digestion of the soil sample to mineralize the n to nh 4, which will be distilled and. copper sulphate acts as a catalyst while potassium sulphate raises the boiling point $$ h_2so_4 $$. such reactions can be significantly sped up by the use of a catalyst and a neutral material like potassium sulphate (\(k_2so_4\)), which raises the reaction’s temperature and the boiling point of the digesting acid. Conversion of nitrogen in the sample to ammonium sulfate. Catalysts like mercury, selenium, copper, or ions of mercury or copper.
from www.slideshare.net
potassium sulphate is usually added to increase the boiling point of the medium. such reactions can be significantly sped up by the use of a catalyst and a neutral material like potassium sulphate (\(k_2so_4\)), which raises the reaction’s temperature and the boiling point of the digesting acid. Conversion of nitrogen in the sample to ammonium sulfate. This method includes a wet digestion of the soil sample to mineralize the n to nh 4, which will be distilled and. The method entails converting all nitrogen in a weighed sample to ammonium sulphate via sulfuric acid digestion, alkalizing the solution, and determining. copper sulphate acts as a catalyst while potassium sulphate raises the boiling point $$ h_2so_4 $$. The acid digestion mixture is diluted and made strongly alkaline with naoh, liberating nh3 as follows:. three basic steps are required to perform the kjeldahl analysis: Catalysts like mercury, selenium, copper, or ions of mercury or copper.
Example Kjeldahl Method
In Kjeldahl's Method Potassium Sulphate Acts As Catalysts like mercury, selenium, copper, or ions of mercury or copper. The acid digestion mixture is diluted and made strongly alkaline with naoh, liberating nh3 as follows:. This method includes a wet digestion of the soil sample to mineralize the n to nh 4, which will be distilled and. Conversion of nitrogen in the sample to ammonium sulfate. copper sulphate acts as a catalyst while potassium sulphate raises the boiling point $$ h_2so_4 $$. three basic steps are required to perform the kjeldahl analysis: potassium sulphate is usually added to increase the boiling point of the medium. Catalysts like mercury, selenium, copper, or ions of mercury or copper. such reactions can be significantly sped up by the use of a catalyst and a neutral material like potassium sulphate (\(k_2so_4\)), which raises the reaction’s temperature and the boiling point of the digesting acid. The method entails converting all nitrogen in a weighed sample to ammonium sulphate via sulfuric acid digestion, alkalizing the solution, and determining.
From slideplayer.com
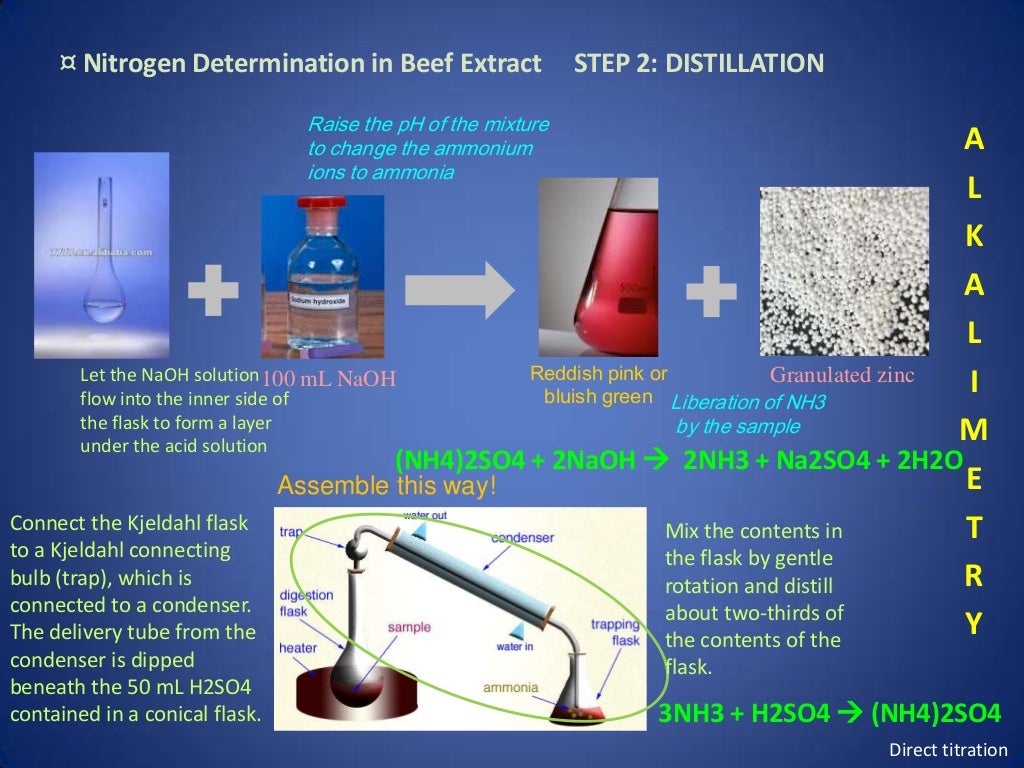
Lab Activity 4 Determination of Nitrogen & Crude Protein ppt download In Kjeldahl's Method Potassium Sulphate Acts As This method includes a wet digestion of the soil sample to mineralize the n to nh 4, which will be distilled and. Catalysts like mercury, selenium, copper, or ions of mercury or copper. Conversion of nitrogen in the sample to ammonium sulfate. such reactions can be significantly sped up by the use of a catalyst and a neutral material. In Kjeldahl's Method Potassium Sulphate Acts As.
From www.scribd.com
Kjeldahl Method PDF Chemical Compounds Chemical Elements In Kjeldahl's Method Potassium Sulphate Acts As The method entails converting all nitrogen in a weighed sample to ammonium sulphate via sulfuric acid digestion, alkalizing the solution, and determining. potassium sulphate is usually added to increase the boiling point of the medium. Conversion of nitrogen in the sample to ammonium sulfate. such reactions can be significantly sped up by the use of a catalyst and. In Kjeldahl's Method Potassium Sulphate Acts As.
From chemistnotes.com
Kjeldahl Method Procedure, Formula and Advantages Chemistry Notes In Kjeldahl's Method Potassium Sulphate Acts As such reactions can be significantly sped up by the use of a catalyst and a neutral material like potassium sulphate (\(k_2so_4\)), which raises the reaction’s temperature and the boiling point of the digesting acid. three basic steps are required to perform the kjeldahl analysis: This method includes a wet digestion of the soil sample to mineralize the n. In Kjeldahl's Method Potassium Sulphate Acts As.
From www.semanticscholar.org
Figure 1 from Is it possible to screen for milk or whey protein In Kjeldahl's Method Potassium Sulphate Acts As such reactions can be significantly sped up by the use of a catalyst and a neutral material like potassium sulphate (\(k_2so_4\)), which raises the reaction’s temperature and the boiling point of the digesting acid. The acid digestion mixture is diluted and made strongly alkaline with naoh, liberating nh3 as follows:. copper sulphate acts as a catalyst while potassium. In Kjeldahl's Method Potassium Sulphate Acts As.
From www.doubtnut.com
In Kjeldahl's method,CuSO(4) acts as In Kjeldahl's Method Potassium Sulphate Acts As The acid digestion mixture is diluted and made strongly alkaline with naoh, liberating nh3 as follows:. potassium sulphate is usually added to increase the boiling point of the medium. This method includes a wet digestion of the soil sample to mineralize the n to nh 4, which will be distilled and. three basic steps are required to perform. In Kjeldahl's Method Potassium Sulphate Acts As.
From www.youtube.com
Kjeldahl's method Estimation of'N' Made easy YouTube In Kjeldahl's Method Potassium Sulphate Acts As Conversion of nitrogen in the sample to ammonium sulfate. Catalysts like mercury, selenium, copper, or ions of mercury or copper. The method entails converting all nitrogen in a weighed sample to ammonium sulphate via sulfuric acid digestion, alkalizing the solution, and determining. The acid digestion mixture is diluted and made strongly alkaline with naoh, liberating nh3 as follows:. three. In Kjeldahl's Method Potassium Sulphate Acts As.
From blogs.thinkmerit.in
Kjeldahl’s method India's Most Reliable Learning App for JEE Main In Kjeldahl's Method Potassium Sulphate Acts As This method includes a wet digestion of the soil sample to mineralize the n to nh 4, which will be distilled and. Conversion of nitrogen in the sample to ammonium sulfate. such reactions can be significantly sped up by the use of a catalyst and a neutral material like potassium sulphate (\(k_2so_4\)), which raises the reaction’s temperature and the. In Kjeldahl's Method Potassium Sulphate Acts As.
From dxoknidin.blob.core.windows.net
Kjeldahl Method Sample Problems at Dorothy Dills blog In Kjeldahl's Method Potassium Sulphate Acts As The acid digestion mixture is diluted and made strongly alkaline with naoh, liberating nh3 as follows:. Conversion of nitrogen in the sample to ammonium sulfate. such reactions can be significantly sped up by the use of a catalyst and a neutral material like potassium sulphate (\(k_2so_4\)), which raises the reaction’s temperature and the boiling point of the digesting acid.. In Kjeldahl's Method Potassium Sulphate Acts As.
From exolntqpa.blob.core.windows.net
Kjeldahl Method Animation at Shaun Abbott blog In Kjeldahl's Method Potassium Sulphate Acts As Conversion of nitrogen in the sample to ammonium sulfate. The method entails converting all nitrogen in a weighed sample to ammonium sulphate via sulfuric acid digestion, alkalizing the solution, and determining. The acid digestion mixture is diluted and made strongly alkaline with naoh, liberating nh3 as follows:. This method includes a wet digestion of the soil sample to mineralize the. In Kjeldahl's Method Potassium Sulphate Acts As.
From www.doubtnut.com
Kjeldahl's method is used in the estimation of In Kjeldahl's Method Potassium Sulphate Acts As Conversion of nitrogen in the sample to ammonium sulfate. three basic steps are required to perform the kjeldahl analysis: The method entails converting all nitrogen in a weighed sample to ammonium sulphate via sulfuric acid digestion, alkalizing the solution, and determining. such reactions can be significantly sped up by the use of a catalyst and a neutral material. In Kjeldahl's Method Potassium Sulphate Acts As.
From www.doubtnut.com
K2SO4 and CuSO4 are added in Kjeldahl's method K2SO4 acts as a catalys In Kjeldahl's Method Potassium Sulphate Acts As such reactions can be significantly sped up by the use of a catalyst and a neutral material like potassium sulphate (\(k_2so_4\)), which raises the reaction’s temperature and the boiling point of the digesting acid. Catalysts like mercury, selenium, copper, or ions of mercury or copper. copper sulphate acts as a catalyst while potassium sulphate raises the boiling point. In Kjeldahl's Method Potassium Sulphate Acts As.
From exoakxsje.blob.core.windows.net
Kjeldahl Setup at John Grise blog In Kjeldahl's Method Potassium Sulphate Acts As The method entails converting all nitrogen in a weighed sample to ammonium sulphate via sulfuric acid digestion, alkalizing the solution, and determining. This method includes a wet digestion of the soil sample to mineralize the n to nh 4, which will be distilled and. three basic steps are required to perform the kjeldahl analysis: The acid digestion mixture is. In Kjeldahl's Method Potassium Sulphate Acts As.
From www.numerade.com
SOLVEDMark the incorrect statement in Kjeldahl's method of estimation In Kjeldahl's Method Potassium Sulphate Acts As Conversion of nitrogen in the sample to ammonium sulfate. such reactions can be significantly sped up by the use of a catalyst and a neutral material like potassium sulphate (\(k_2so_4\)), which raises the reaction’s temperature and the boiling point of the digesting acid. potassium sulphate is usually added to increase the boiling point of the medium. three. In Kjeldahl's Method Potassium Sulphate Acts As.
From testbook.com
Kjeldahl Method Steps, Formula, Principle, Calculation & Procedure In Kjeldahl's Method Potassium Sulphate Acts As potassium sulphate is usually added to increase the boiling point of the medium. This method includes a wet digestion of the soil sample to mineralize the n to nh 4, which will be distilled and. such reactions can be significantly sped up by the use of a catalyst and a neutral material like potassium sulphate (\(k_2so_4\)), which raises. In Kjeldahl's Method Potassium Sulphate Acts As.
From www.slideserve.com
PPT PROTEIN ANALYSIS PowerPoint Presentation, free download ID2060791 In Kjeldahl's Method Potassium Sulphate Acts As The acid digestion mixture is diluted and made strongly alkaline with naoh, liberating nh3 as follows:. This method includes a wet digestion of the soil sample to mineralize the n to nh 4, which will be distilled and. three basic steps are required to perform the kjeldahl analysis: The method entails converting all nitrogen in a weighed sample to. In Kjeldahl's Method Potassium Sulphate Acts As.
From www.youtube.com
Problem based on Kjeldahls method...11th chemistry in தமிழ்... ☺ YouTube In Kjeldahl's Method Potassium Sulphate Acts As Catalysts like mercury, selenium, copper, or ions of mercury or copper. Conversion of nitrogen in the sample to ammonium sulfate. copper sulphate acts as a catalyst while potassium sulphate raises the boiling point $$ h_2so_4 $$. potassium sulphate is usually added to increase the boiling point of the medium. The method entails converting all nitrogen in a weighed. In Kjeldahl's Method Potassium Sulphate Acts As.
From exolntqpa.blob.core.windows.net
Kjeldahl Method Animation at Shaun Abbott blog In Kjeldahl's Method Potassium Sulphate Acts As Catalysts like mercury, selenium, copper, or ions of mercury or copper. This method includes a wet digestion of the soil sample to mineralize the n to nh 4, which will be distilled and. three basic steps are required to perform the kjeldahl analysis: potassium sulphate is usually added to increase the boiling point of the medium. The acid. In Kjeldahl's Method Potassium Sulphate Acts As.
From askfilo.com
(ii) Kjeldahl's method The compound containing nitrogen is heated with c.. In Kjeldahl's Method Potassium Sulphate Acts As The method entails converting all nitrogen in a weighed sample to ammonium sulphate via sulfuric acid digestion, alkalizing the solution, and determining. Conversion of nitrogen in the sample to ammonium sulfate. The acid digestion mixture is diluted and made strongly alkaline with naoh, liberating nh3 as follows:. potassium sulphate is usually added to increase the boiling point of the. In Kjeldahl's Method Potassium Sulphate Acts As.